The framework of UniBioPAN.
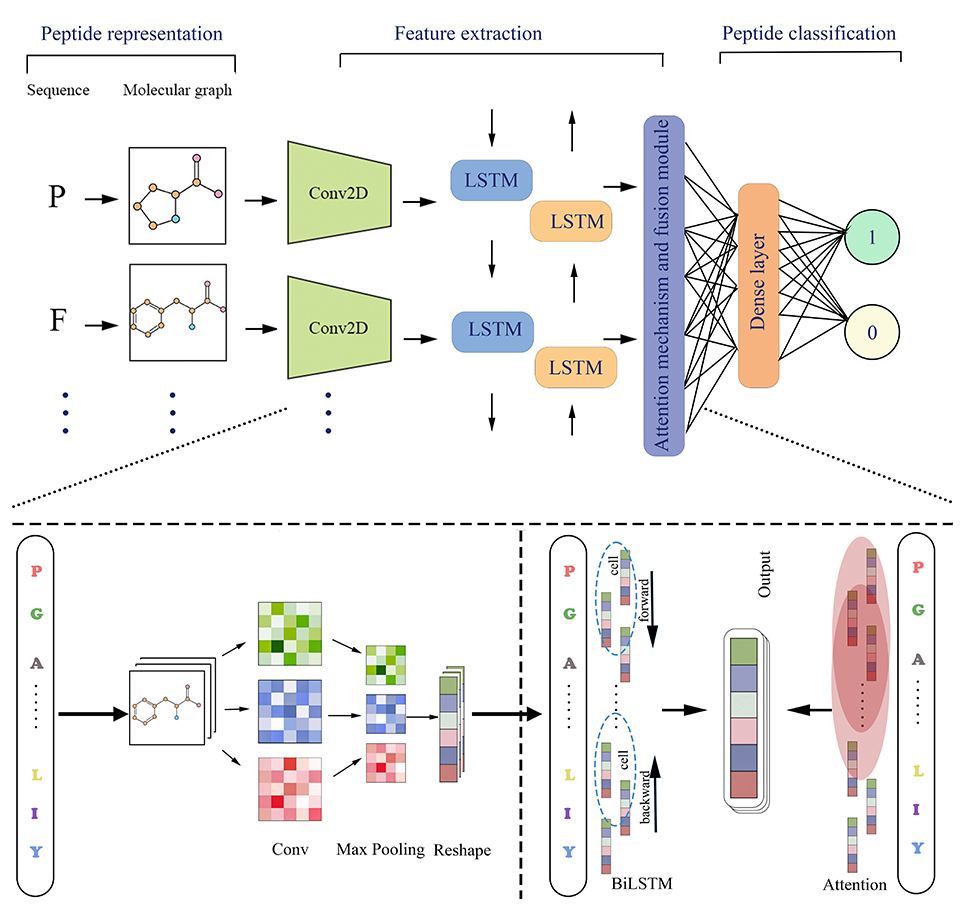
Figure 1.The framework of model
Peptide representation:
Our approach to peptide characterization draws inspiration from video classification methodologies. We model each amino acid residue as a unique molecular image, encoding a wealth of chemical information, such as atoms, chemical bonds, functional groups, and other relevant structural attributes. This innovative framework conceptualizes peptides as dynamic video, the researchers can view the detailed information of each peptide as coherently and comprehensively as if watching a video. . This representation enables neural networks to seamlessly and automatically extract intricate features from the molecular graphs. This facilitates the capture of nuanced patterns within the peptide sequences and empowers the model to discern salient characteristics crucial for effective representation and subsequent analysis. In essence, we transform the sequential nature of amino acid sequences into a visual representation, mirroring the temporal evolution captured in videos. This paradigm shift allows us to leverage sophisticated neural network architectures for comprehensive feature extraction, ultimately leading to enhanced understanding and classification of bioactive peptides.
Feature extraction:
Amino acid sequences undergo a transformation into molecular image, and the subsequent extraction of features is accomplished through Conv2D convolutional layers. 2D CNN can effectively recognize local features in images and transform them into high-dimensional feature vectors . To capture the contextual relationships between sequences, BiLSTM was employed to extract sequence features. BiLSTM can learn the forward and backward information of the sequence, thereby better understanding the overall meaning of the sequence. To focus on crucial residues in the sequence, an attention mechanism layer was incorporated. The attention mechanism can assign different weights to residues according to their importance, thereby improving the model's performance and generalization. This fusion of Conv2D, BiLSTM, and attention mechanisms empowers the model to distill essential features from amino acid sequences, contributing to a robust and nuanced representation
Peptide classification:
The extracted features are subsequently fed into fully connected layers for classification. This enables the model to leverage the comprehensively distilled features to make informed decisions and achieve precise categorization. This end-to-end approach, encompassing feature extraction and classification, ensures thorough and effective processing of amino acid sequences, allowing the model to accurately discern and classify bioactive peptides. The architecture of the feature extraction component is illustrated in Figure 1.
The biological functions of peptides
Antihypertensive peptides:
Anti-hypertensive peptides are peptides that have the ability to lower blood pressure. They can be extracted from food, animal proteins, or plant proteins.
Mechanisms of Action:
1. Inhibition of Angiotensin-Converting Enzyme (ACE) Activity: ACE is an enzyme that regulates blood vessel constriction and blood pressure. Anti-hypertensive peptides can inhibit the activity of ACE, thus reducing the likelihood of blood vessel constriction and elevated blood pressure.
2. Promotion of Nitric Oxide (NO) Release: NO is an important vasodilator, and anti-hypertensive peptides can promote its release, causing blood vessels to relax and lowering blood pressure.
3. Antioxidant Activity: Some anti-hypertensive peptides have antioxidant activity, reducing the production of free radicals and protecting blood vessel walls from oxidative damage.
4. Inhibition of Sodium Absorption: Some anti-hypertensive peptides may reduce blood volume by inhibiting sodium absorption in the kidneys, thereby lowering blood pressure.
5. Inhibition of the Renin-Angiotensin System: Anti-hypertensive peptides may lower blood pressure by inhibiting the activity of the renin-angiotensin system.
DPP-IV inhibitors peptides:
DPP-IV inhibitors peptides refer to a class of peptides that inhibit the activity of the enzyme dipeptidyl peptidase-4 (DPP-IV). DPP-IV is an enzyme that breaks down incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These hormones play a crucial role in regulating blood sugar levels by stimulating insulin secretion from pancreatic beta cells and inhibiting glucagon secretion from pancreatic alpha cells after meals.
Bitter peptides:
Bitter peptides are a structurally diverse group of oligopeptides often generated in fermented, aged, and hydrolyzed food products that make them unfavorable for consumption. Humans perceive bitterness by a repertoire of 25 human bitter receptors, termed T2Rs[1].
[1] Maehashi, K., and Liquan Huang. "Bitter peptides and bitter taste receptors." Cellular and molecular life sciences 66 (2009): 1661-1671.
Umami peptides:
Umami peptides are a type of peptide substances with umami characteristics, which are the fourth type of umami substance discovered after monosodium glutamate, inosinic acid, and guanylic acid. Umami peptides are widely present in natural foods such as meat, fish, vegetables, dairy products, etc., and can also be obtained through chemical synthesis or enzymatic hydrolysis. The amino acid composition and conformation of umami peptides are crucial factors determining their umami characteristics.
Peptides containing amino acids such as glutamate, aspartate, phenylalanine, and tyrosine typically exhibit umami taste. Umami peptides can be used to produce umami enhancers, enhancing the umami taste of food, and replacing traditional chemically synthesized umami enhancers.
Umami peptides have good nutritional value and can be used for the development of functional foods.
Anti-microbial peptides:
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria[1], enveloped viruses, fungi and even transformed or cancerous cells[2]. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.
[2] Reddy, K. V. R., R. D. Yedery, and C. Aranha. "Antimicrobial peptides: premises and promises." International journal of antimicrobial agents 24.6 (2004): 536-547.
Anti-malarial peptides:
Antimalarial peptides are a class of peptide molecules with antimalarial activity, representing an emerging field in malaria treatment research in recent years. These peptides can inhibit the growth and reproduction of malaria parasites through various mechanisms, including disrupting the cell membrane of the parasites, interfering with intracellular biochemical processes, or activating the immune system to combat the parasites.
Quorum sensing peptides:
Quorum sensing peptides (QSPs) are microbial signaling molecules involved in various cellular processes such as cell communication, virulence expression, bioluminescence, and swarm behavior. Understanding QSPs is crucial for identifying new drug targets to control bacterial populations and pathogenicity.
Anti-cancer peptides:
Anti-cancer peptides (ACPs) represent a diverse array of short peptide sequences renowned for their remarkable ability to impede various facets of cancer progression. These peptides exhibit multifaceted actions, encompassing the inhibition of tumor cell proliferation and migration, as well as the suppression of tumor angiogenesis, thereby hindering the formation of new blood vessels crucial for tumor sustenance. Notably, ACPs possess a distinct advantage over conventional cancer therapies, as they are less prone to eliciting drug resistance, a persistent challenge in cancer treatment. Owing to these inherent merits, ACPs emerge as exceptionally promising candidates in the quest for effective anti-cancer interventions.
Anti-MRSAs trains peptides:
Anti-MRSA peptides, especially those targeting methicillin-resistant Staphylococcus aureus (MRSA) strains, have garnered significant attention due to their rapid and broad-spectrum antibacterial activity. As conventional antibiotics face increasing resistance from bacteria, antimicrobial peptides have emerged as potential therapies for bacterial infections. Anti-MRSA peptides represent a class of promising treatment options capable of addressing various infections caused by MRSA. These peptides penetrate bacterial cell membranes and interact with their internal structures, inhibiting their growth and proliferation. With their specificity and relatively low toxicity, anti-MRSA peptides are considered a more sustainable and effective antibacterial treatment, offering potential solutions to the challenges posed by antibiotic-resistant bacterial infections in the future.
Tumor T cell antigens peptides:
Tumor T cell antigen peptides, also known as tumor-associated antigens (TAAs) or tumor-specific antigens, are short peptide sequences derived from proteins that are uniquely expressed or overexpressed in tumor cells. These peptides can elicit immune responses from T cells, which are a type of white blood cell crucial for immune defense against cancer.
Tumor T cell antigen peptides are recognized by T cells through their T cell receptors (TCRs) when presented on the surface of cancer cells by major histocompatibility complex (MHC) molecules. This recognition triggers an immune response against the tumor cells, leading to their destruction.
These peptides hold significance in cancer immunotherapy as they can be utilized to develop vaccines or adoptive cell therapies aimed at boosting the body's immune response against cancer. Additionally, they are used in research to better understand the immune response to tumors and to identify potential targets for immunotherapy.
Blood-Brain Barrier peptides:
Blood-brain barrier (BBB) peptides are short peptide sequences that have the ability to cross the blood-brain barrier, a highly selective semipermeable membrane that separates the circulating blood from the brain extracellular fluid in the central nervous system (CNS). The BBB plays a crucial role in protecting the brain by regulating the passage of substances between the bloodstream and the brain.
BBB peptides are designed or selected for their capacity to traverse this barrier. They can be utilized for various purposes, including drug delivery to the brain for the treatment of neurological disorders such as brain tumors, Alzheimer's disease, Parkinson's disease, and others. These peptides can be conjugated to drugs or nanoparticles to facilitate their transport across the BBB, thereby improving the efficacy of therapeutic agents in treating CNS disorders. Additionally, BBB peptides can serve as research tools for studying the physiology of the blood-brain barrier and developing strategies to overcome its limitations in drug delivery to the brain.
Anti-parasitic peptides:
Anti-parasitic peptides are short peptide sequences that exhibit activity against parasitic organisms. These peptides can be naturally occurring or synthetic and are designed to disrupt essential biological processes in parasites, ultimately leading to their death or inhibition of their growth and reproduction.
Anti-parasitic peptides work through various mechanisms, including disrupting the integrity of the parasite's cell membrane, interfering with its metabolic pathways, or targeting specific molecular components essential for its survival. They may also modulate the host immune response to enhance parasite clearance.
These peptides hold promise as alternative or adjunctive therapies for treating parasitic infections, including those caused by protozoa, helminths, and ectoparasites. With the increasing emergence of drug-resistant parasites and the limitations of current treatment options, anti-parasitic peptides offer a potential solution for combating parasitic diseases effectively. Additionally, their specificity and relatively low toxicity make them attractive candidates for further development as anti-parasitic drugs.
Neuro peptide peptides:
Neuropeptide peptides are short peptide sequences that act as signaling molecules in the nervous system. They are produced and released by neurons and modulate various physiological processes, including neurotransmission, neuronal development, pain perception, mood regulation, and hormone secretion.
Neuropeptides function by binding to specific receptors on target cells, triggering a cascade of cellular events that regulate neuronal activity or influence other physiological functions. They are involved in complex neural networks and contribute to the regulation of diverse physiological and behavioral processes.
Examples of neuropeptide peptides include substance P, oxytocin, vasopressin, endorphins, and neuropeptide Y, among others. These peptides play crucial roles in the central and peripheral nervous systems and are implicated in various neurological and psychiatric disorders. Studying neuropeptides and their interactions with receptors is essential for understanding brain function and developing therapeutic interventions for neurological and neuropsychiatric conditions.
Anti-bacterial peptides:
Anti-bacterial peptides, also known as antimicrobial peptides (AMPs), are short peptide sequences that exhibit activity against bacteria. These peptides are naturally produced by a wide range of organisms, including plants, animals, and microorganisms, as part of their innate immune defense mechanisms.
Anti-bacterial peptides function by disrupting bacterial cell membranes, interfering with essential cellular processes, or targeting specific intracellular components of bacteria. They exhibit broad-spectrum activity against a variety of bacterial species, including both Gram-positive and Gram-negative bacteria. Their potential applications include the treatment of bacterial infections, the prevention of bacterial colonization on medical devices and surfaces, and the enhancement of food preservation techniques.
Anti-fungal peptides:
Anti-fungal peptides, also known as antifungal peptides (AFPs), are short peptide sequences that exhibit activity against fungal pathogens. They are naturally produced by various organisms as part of their innate immune defense mechanisms.
AFPs function by disrupting fungal cell membranes, interfering with essential cellular processes, or targeting specific intracellular components of fungi. They possess broad-spectrum activity against a wide range of fungal species, including yeasts and molds.
These peptides play a crucial role in host defense against fungal infections and are being explored as potential candidates for the development of new antifungal agents. Their applications include the treatment of fungal infections in humans, animals, and plants, as well as the prevention of fungal growth on surfaces and in food products.
Similar to anti-bacterial peptides, anti-fungal peptides offer advantages over conventional antifungal drugs, including their rapid action, low propensity for inducing resistance in fungi, and potential synergistic effects with existing antifungal agents.
Anti-angiogenic peptides:
Anti-angiogenic peptides function by interfering with the signaling pathways involved in blood vessel formation or by directly targeting endothelial cells, which line the interior of blood vessels. By inhibiting angiogenesis, these peptides can prevent the growth and spread of tumors by depriving them of the blood supply they need to thrive.
These peptides hold promise as potential therapeutics for cancer and other angiogenesis-dependent diseases. They can be administered alone or in combination with other anti-cancer therapies to enhance their efficacy. Additionally, anti-angiogenic peptides are being investigated for their potential to treat ocular diseases, cardiovascular disorders, and other conditions characterized by abnormal angiogenesis.
Toxicity peptides:
Therapeutic Peptides with Toxic Side Effects: Some therapeutic peptides, while designed to have beneficial effects, may exhibit unintended toxic side effects in certain individuals or at high doses.
Traditional wet lab methods for peptide toxicity assessment are time-consuming and costly. In vitro cell culture assays and in vivo animal studies, while valuable, require specialized facilities and expertise. Moreover, they may not fully capture all toxic effects. Thus, there's interest in alternative approaches like computational modeling to expedite toxicity evaluation in drug development.
Anti-inflammatory peptides:
Anti-inflammatory peptides are short sequences of amino acids that possess the ability to reduce inflammation in the body. They work by modulating the activity of inflammatory mediators, such as cytokines, chemokines, and enzymes involved in the inflammatory response.
These peptides can be derived from various sources, including food proteins, microbial organisms, and synthetic design. They exert their anti-inflammatory effects through multiple mechanisms, such as inhibiting the production of pro-inflammatory cytokines, suppressing the activation of inflammatory signaling pathways, and promoting the release of anti-inflammatory molecules.
Anti-inflammatory peptides have been studied for their potential therapeutic applications in various inflammatory conditions, including arthritis, inflammatory bowel disease, asthma, and dermatitis. They offer several advantages over traditional anti-inflammatory drugs, such as reduced side effects, increased bioavailability, and potential for targeted delivery to inflamed tissues.
Pro-inflammatory peptides:
Pro-inflammatory peptides refers to short sequences of amino acids that have the opposite effect of anti-inflammatory peptides. These peptides promote or exacerbate inflammation in the body by stimulating the production or activity of inflammatory mediators, such as cytokines, chemokines, and prostaglandins.
Pro-inflammatory peptides can be produced endogenously in the body in response to various stimuli, such as injury, infection, or stress. They can also be derived from external sources, such as dietary proteins or microbial pathogens.
These peptides play a role in the initiation and propagation of inflammatory responses, which are essential for the body's defense against pathogens and tissue damage. However, excessive or prolonged inflammation can contribute to the pathogenesis of various diseases, including autoimmune disorders, chronic inflammatory conditions, and cardiovascular diseases.
IL-6 inducing peptides:
IL-6 inducing peptides are short peptide sequences that stimulate the production or release of interleukin-6 (IL-6) in the body. Interleukin-6 is a cytokine involved in various physiological processes, including inflammation, immune response, and hematopoiesis.
Peptides that induce IL-6 production can have different sources and mechanisms of action. They may be derived from proteins naturally present in the body or synthesized in the laboratory. These peptides can interact with specific receptors on immune cells or other cell types, triggering intracellular signaling pathways that lead to the synthesis and secretion of IL-6.
IL-6 inducing peptides have implications in various areas of research and medicine. In the context of inflammation and immune response, they can be used to study the regulation of IL-6 production and its role in inflammatory diseases, autoimmune disorders, and cancer. Additionally, IL-6 inducing peptides may have therapeutic applications, such as in immunotherapy for cancer or as adjuvants to enhance the efficacy of vaccines.
Anti-tubercular peptides:
Anti-tubercular peptides are short sequences of amino acids that exhibit activity against Mycobacterium tuberculosis, the bacterium responsible for causing tuberculosis (TB). These peptides are being investigated as potential therapeutic agents for the treatment of TB, particularly in light of the increasing prevalence of drug-resistant strains of M. tuberculosis.
Anti-tubercular peptides can exert their antimicrobial effects through various mechanisms, including disrupting the bacterial cell membrane, interfering with essential metabolic pathways, or targeting specific bacterial proteins or enzymes critical for survival and replication. These peptides may also modulate the host immune response to enhance the clearance of M. tuberculosis infection.
Cell-penetrating peptides:
Cell-penetrating, also known as cell-penetrating peptides (CPPs) or protein transduction domains (PTDs), refers to short peptide sequences that have the ability to cross cell membranes and enter cells efficiently. These peptides are typically composed of fewer than 30 amino acids and are characterized by their ability to traverse cellular membranes, which are otherwise impermeable to large molecules.
Once inside cells, cell-penetrating peptides can deliver a variety of cargo molecules, including small molecules, nucleic acids (e.g., DNA, RNA), proteins, peptides, and nanoparticles. This ability to deliver cargo across cell membranes has led to the development of cell-penetrating peptide-based technologies for drug delivery, gene therapy, and molecular imaging.
Cell-penetrating peptides hold significant promise for biomedical applications due to their ability to overcome one of the major barriers in drug delivery: the impermeability of cell membranes. However, challenges remain in optimizing the specificity, efficiency, and safety of cell-penetrating peptide-based delivery systems for various therapeutic and diagnostic applications.
Anti-oxidant peptides:
Anti-oxidant peptides are short sequences of amino acids that exhibit antioxidant activity. Antioxidants are compounds that help neutralize harmful free radicals in the body, which are byproducts of normal cellular metabolism and can cause oxidative damage to cells and tissues. Oxidative stress, resulting from an imbalance between the production of free radicals and the body's ability to neutralize them, is implicated in various diseases, including cancer, cardiovascular disease, and neurodegenerative disorders.
Tumor-Homing Peptides peptides:
Tumor-homing peptides are short sequences of amino acids that have a specific affinity for tumor cells or their microenvironment. These peptides possess the ability to selectively bind to molecular targets that are overexpressed or uniquely expressed on the surface of tumor cells or within the tumor microenvironment. By exploiting the unique characteristics of tumors, such as their altered cell surface receptors or extracellular matrix components, tumor-homing peptides can serve as targeting ligands for various applications in cancer diagnosis, imaging, and therapy.
Tumor-homing peptides can be utilized for targeted drug delivery, where therapeutic agents such as chemotherapy drugs, imaging agents, or therapeutic nanoparticles are conjugated to the peptides. This targeted approach aims to enhance the accumulation of therapeutic or diagnostic agents within tumor tissues while minimizing systemic side effects on healthy tissues.
Additionally, tumor-homing peptides can be used for molecular imaging of tumors, allowing for non-invasive detection and visualization of tumor lesions. By conjugating imaging probes to tumor-homing peptides, researchers can track the location, size, and extent of tumor growth in vivo, aiding in cancer diagnosis, staging, and treatment monitoring.
Viral integrase inhibitory peptides:
Viral integrase inhibitory peptides are short sequences of amino acids that possess the ability to inhibit the activity of viral integrase enzymes. Integrase is an enzyme produced by retroviruses, including HIV, and some other viruses like hepatitis B virus (HBV), which plays a crucial role in the viral replication cycle.
The main function of viral integrase is to catalyze the integration of viral DNA into the host cell genome, a critical step in the viral replication process. By inhibiting integrase activity, viral integrase inhibitory peptides can prevent the integration of viral DNA into the host cell genome, thereby blocking viral replication and reducing viral load.
Viral integrase inhibitory peptides hold promise as potential antiviral therapeutics for the treatment of retroviral infections, such as HIV and HBV. They offer advantages such as specificity for viral targets, low toxicity, and potential for use in combination with other antiviral drugs to enhance efficacy and reduce the risk of drug resistance.
Anti-diabetic peptides:
Anti-diabetic peptides are short sequences of amino acids that exhibit potential in managing diabetes mellitus, a chronic metabolic disorder characterized by high blood sugar levels. These peptides may offer therapeutic benefits by modulating various pathways involved in glucose metabolism, insulin secretion, insulin sensitivity, and inflammation.
Anti-diabetic peptides can be derived from natural sources, such as food proteins (e.g., milk, eggs, soy), plant extracts, or animal tissues, or they can be designed or synthesized using computational methods. They may exert their effects through multiple mechanisms, including:
1. Inhibition of enzymes involved in carbohydrate digestion and absorption, such as α-amylase and α-glucosidase, leading to reduced postprandial blood glucose levels.
2. Enhancement of insulin secretion from pancreatic β-cells or improvement of β-cell function.
3. Enhancement of insulin sensitivity in peripheral tissues, such as skeletal muscle and adipose tissue.
4. Modulation of inflammatory pathways associated with insulin resistance and β-cell dysfunction.
5. Protection of pancreatic β-cells from oxidative stress and apoptosis.
IL-5 inducing:
AIL-5 inducing peptide is a peptide that can stimulate and increase the production of interleukin-5 (IL-5). IL-5 is a cytokine primarily produced by T lymphocytes and mast cells. It acts on eosinophils, promoting their growth, differentiation, recruitment, and activation. Eosinophils play a significant role in immune responses, particularly in allergic reactions and asthma.
Mechanism of Action of IL-5 Inducing Peptides:
1. IL-5 inducing peptides can bind to specific cell surface receptors, initiating cellular signaling pathways.
2. This binding activates intracellular signaling pathways, typically involving molecules such as Janus kinase (JAK) and signal transducers and activators of transcription (STAT).
3. The activation of signaling pathways leads to an increase in the transcription and expression of the IL-5 gene, resulting in the production of more IL-5.
IL-13 inducing:
IL-13 inducing peptides are peptides that can stimulate and increase the production of interleukin-13 (IL-13). IL-13 is a cytokine primarily produced by T helper type 2 (Th2) cells, mast cells, and some other immune cells. It plays a crucial role in the regulation of inflammatory and immune responses, particularly in allergic reactions and asthma.
Mechanism of Action of IL-13 Inducing Peptides:
1. IL-13 inducing peptides bind to specific cell surface receptors, which could include receptors on Th2 cells or other immune cells.
2. These peptides trigger intracellular signaling pathways upon receptor binding, often involving molecules like Janus kinase (JAK) and signal transducers and activators of transcription (STAT).
Biofilm inhibitory:
biofilm inhibitory peptide is a type of peptide that can prevent or disrupt the formation of biofilms. Biofilms are structured communities of microorganisms, such as bacteria or fungi, that are attached to a surface and encased in a self-produced extracellular matrix. This matrix protects the microorganisms from environmental stress and antimicrobial agents, making biofilms a significant challenge in medical, industrial, and environmental contexts.
Mechanisms of Biofilm Inhibition by Peptides:
1. Some peptides can prevent the initial attachment of microorganisms to surfaces, thereby inhibiting the formation of biofilms. They achieve this by disrupting the cell surface structures or signaling pathways that mediate attachment.
2. Biofilm inhibitory peptides can degrade or interfere with the production of the extracellular matrix that encases the microorganisms. This can weaken the biofilm structure and make the microorganisms more susceptible to antimicrobial agents.
3. Quorum sensing is a communication process used by bacteria to coordinate their behavior in response to population density. Some peptides can interfere with quorum sensing signals, preventing the bacteria from initiating biofilm formation or coordinating biofilm-related activities.
4. Many biofilm inhibitory peptides possess antimicrobial properties, meaning they can kill or inhibit the growth of microorganisms directly. By reducing the number of viable cells, these peptides can prevent the establishment and growth of biofilms.
Hemolytic:
Hemolytic peptides are a class of peptides that can cause the lysis (destruction) of red blood cells (erythrocytes). These peptides disrupt the cell membrane, leading to the release of hemoglobin and other intracellular contents into the surrounding environment. Hemolytic peptides can be naturally occurring or synthetically designed, and they are often characterized by their ability to interact with and disrupt lipid bilayers.
Hemolytic peptides are studied for their antimicrobial properties. While their hemolytic activity can be a drawback for therapeutic use, understanding their mechanism can help in designing non-hemolytic antimicrobial peptides.
Some hemolytic peptides exhibit selective toxicity towards cancer cells. Researchers explore their potential as therapeutic agents that can target and destroy cancer cells while minimizing damage to healthy cells.